Abstract
Background
Decreased immune response to vaccinations has been a long-standing concern for patients with chronic lymphocytic leukemia (CLL), particularly those receiving B-cell directed therapy. We have previously demonstrated the negative impact of Bruton tyrosine kinase (BTK) and B-cell lymphoma 2 (BCL-2) inhibitors on COVID vaccine efficacy for those with CLL with an overall seroconversion rate of 41% (Ujjani et al, BJH 2022). Here we present the 1-year follow up data of from our cohort.
Methods
This prospective observational study was conducted at the University of Washington/Fred Hutchinson Cancer Center. Eligible patients had a diagnosis of CLL or SLL, were at least 18-years-old and had no known prior history of SARS-CoV-2 infection. Patients were enrolled into 3 cohorts: 1) currently receiving or recently completed venetoclax + anti-CD20 monoclonal antibody (Arm A), 2) currently receiving a BTK inhibitor (Arm B), and 3) previously untreated (Arm C). Patients underwent testing prior to vaccination (Visit 1), 1 (Visit 2), 3 (Visit 3), 6 (Visit 4), and 12 (Visit 5) months after first vaccine. Vaccine response was assessed via the Roche Elecsys Anti-SARS-CoV-2 S, a semiquantitative total antibody assay against the spike protein receptor binding domain (anti-S) and SARS-CoV-2 spike D614G pseudotyped lentivirus neutralization assays. We have previously demonstrated that these assays perform similarly with non-significant discordance, (p = 0.617). Reference interval for a negative result was <0.8 AU/ml on the Roche anti-S assay and ND50 < 20 for the neutralization assay.
Results
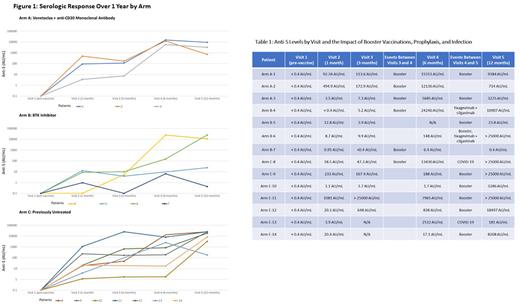
Of the original cohort of 37 evaluable patients, 14 remain on study at 1 year of follow up. The median age was 67 years (range 42-77) and 9 were male (64%). The median IgG level was 657 mg/dL (range 214-1016). Ten received BNT162b2 (72%), 3 mRNA-1273 (22%), and 1 Ad26.COV2.S (6%). Patient enrollment by arm was Arm A (n=3), Arm B (n=4), Arm C (n=7). All of the patients in Arm A had recently completed venetoclax therapy prior to first vaccination, whereas all of the patients in Arm B remained on a BTK inhibitor during the observation period of this study. None of the patients in any of the arms initiated a new line of therapy before Visit 5. Of the 6 patients who had received any prior anti-CD20 monoclonal antibody, all had received it more than 1 year prior to first vaccination (range 13-106 months). Anti-nucleocapsid antibody was non-reactive in all patients, indicating no evidence of prior SARS-CoV-2 infection.
Median Anti-S levels at Visit 2 were 92.3 AU/mL (3.47-494) in Arm A, 8.7 AU/mL (0.95-12.8) in Arm B, and 20.1 AU/mL (1.14-1081) in Arm C. Anti-S levels from Visits 1-5 are depicted in Figure 1 by Arm. Variables that may have impacted serologic response, such as booster vaccinations, prophylactic tixagevimab with cilgavimab, COVID-19 infections, are depicted in Table 1. All 6 patients who received a booster after Visit 3 (3 months) were noted to have an increase in Anti-S by the Visit 4 (6 months) assessment. The increase was noted in 3 patients in Arm A, 1 patient in Arm B, and 1 patient in Arm C. Of the 9 patients who received a booster after Visit 4, 7 were noted to have increased Anti-S levels by the Visit 5 (12 months) assessment. There was no consistent trend for increased Anti-S levels after prophylactic tixagevimab with cilgavimab or COVID-19 infection.
Roche Anti-S testing at Visit 5 showed similar results to neutralization antibody testing. The only discrepancy was noted in a patient with a minor, but technically positive, Anti-S level of 23.8 AU/mL and negative neutralization results.
Two COVID-19 infections were noted (Table 1). Both were uncomplicated courses in previously untreated patients who had robust serologic responses to the vaccine. The infections occurred between Visits 4 and 5, during the Omicron variant phase but sequencing data was not available. Neither patient had received prophylactic tixagevimab with cilgavimab. One of these patients had received one dose of Ad26.COV2.S, whereas the other had received 3 doses of BNT162b2.
Conclusion
Here, we show that while CLL patients typically have inferior serologic responses to initial COVID vaccinations, these responses can be augmented with the administration of booster vaccinations. Benefit was primarily noted in patients who had completed venetoclax therapy and those who were previously untreated. Patients with CLL remain a vulnerable population as new variants arise.
Disclosures
Ujjani:Incyte: Honoraria; Janssen: Consultancy; Beigene: Consultancy; Abbvie: Consultancy, Research Funding; TG Therapeutics: Honoraria; Genentech: Consultancy; Eli Lilly: Honoraria, Research Funding; epizyme: Consultancy; Atara: Consultancy; astrazeneca: Consultancy, Research Funding; morphosys: Honoraria; Pharmacyclics: Consultancy, Research Funding. Poh:Incyte Morphosys: Consultancy, Research Funding. Shadman:Regeneron: Consultancy; Merck: Consultancy; Fate Therapeutics: Consultancy; MEI Pharma: Consultancy; Atara Biotherapeutic: Consultancy, Research Funding; Adaptimmune: Consultancy; Innate Pharma: Consultancy; Mustang Bio: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy; Kite Pharma: Consultancy; Pharmacyclics: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Morphosys/Incyte: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Epizyme: Consultancy; Epi Lilly: Consultancy; AstraZeneca: Consultancy, Research Funding; Sound Biologics: Consultancy; Genentech: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Celgene, a BMS Company: Research Funding; Gilead: Research Funding; Sunesis: Research Funding; Genmab: Research Funding. Lynch:TG Therapeutics: Research Funding; Incyte: Research Funding; Bayer: Research Funding; Cyteir: Research Funding; Genentech: Research Funding; Seagen: Research Funding; Rapt: Research Funding; Cancer Study Group: Consultancy. Gopal:Merck, I-Mab bio, IgM Bio, Takeda, Gilead, Astra-Zeneca, Agios, Janssen, BMS, SeaGen, Teva, Genmab: Research Funding; Incyte, Kite, Morphosys/Incyte, ADCT, Acrotech, Merck, Karyopharm, Servier, Beigene, Cellectar, Janssen, SeaGen, Epizyme, I-Mab bio, Gilead, Genentech, Lilly, Caribou, Fresenius-Kabi: Consultancy, Honoraria. Till:Proteios Technology: Consultancy; BMS/Celgene: Research Funding; Mustang Bio: Consultancy, Patents & Royalties, Research Funding. Smith:Abbvie: Consultancy; ADC Therapeutics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Bayer: Research Funding; Beigene: Consultancy, Research Funding; De Novo Biopharma: Research Funding; Enterome: Research Funding; Epizyme: Consultancy; Genentech: Research Funding; Incyte Corporation: Consultancy, Research Funding; Karyopharm: Consultancy; KITE pharma: Consultancy; Kymera Therapeutics: Research Funding; Merck Sharp/Dohme Corp: Research Funding; MorphoSys: Research Funding; Nanjing Pharmaceuticals Co., Ltd: Research Funding; Numab Therapeutics AG: Consultancy; Portola Pharmaceuticals: Research Funding; Viracta Therapeutics: Research Funding. Hill:Karius: Research Funding; Optum Health: Consultancy; CLS Behring: Consultancy; Takeda: Consultancy, Research Funding; AlloVir: Consultancy, Research Funding; Octapharma: Consultancy; Gilead: Consultancy, Research Funding; CRISPR Therapeutics: Consultancy; Allogene Therapeutics: Consultancy; Amplyx: Consultancy. Greninger:Gilead: Research Funding; Merck: Research Funding; LabCorp: Other: Spouse's salary; Abbott: Other: central testing contracts.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal